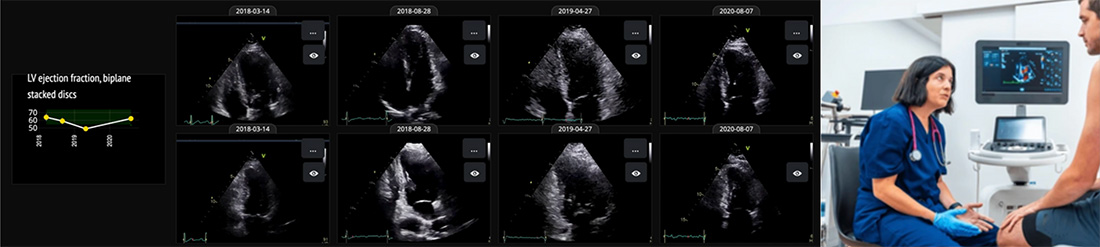
Echo Confidence Receives FDA 510(K) Clearance
Posted on: 04/07/2025
EchoConfidence Receives FDA 510(k) Clearance
We’re thrilled to share a major milestone at MyCardium AI, our AI-powered echocardiography platform, EchoConfidence, has officially received FDA 510(k) clearance!
This clearance not only confirms the safety and effectiveness of EchoConfidence, but it also paves the way for the platform’s clinical use in the United States, bringing explainable, AI-driven echocardiography into routine practice.

What Does This Mean for Clinicians?
EchoConfidence was built with and for the clinical community. It provides:
- Fully automated measurements of cardiac chambers, valves, vessels, and pericardium
- Explainable AI: Every fiducial point, curve, and classification is transparent and editable
- Accelerated workflows with expert-level consistency
- Seamless integration into echo labs, clinical trials, and core labs
Clinically Validated, Expert Trusted
Our AI has been:
- Trained on 100,000+ echo images from over 20,000 patients
- Validated against six international expert readers
- Benchmarked using Mean Absolute Error (MAE) to match inter-expert consistency
- Reviewed using unseen datasets labelled by 8–15 certified cardiologists and sonographers
This ensures that EchoConfidence performs at the level of human experts, while supporting scalable, high-quality cardiac imaging.

“We are pleased to announce that EchoConfidence has received FDA 510(k) clearance, paving the way for clinicians across the United States to access this innovative, AI-powered solution for echocardiography analysis. Developed by cardiologists with deep insight into today’s clinical challenges, EchoConfidence delivers an end-to-end workflow solution designed to transform echo service delivery. EchoConfidence offers over 40% improvement in measurement precision and significantly enhances workflow efficiency, empowering clinicians to deliver high-quality care with greater confidence and consistency.
This achievement reflects the dedication of our world-class team of clinicians, engineers, and researchers, whose commitment to innovation is driving the future of echocardiography. We look forward to continuing to push boundaries and shape the next generation of cardiac care.”
We’re Ready to Partner
Whether you're a hospital looking to streamline echocardiography, a pharma company running trials, or a core lab seeking reproducible data, EchoConfidence is ready to deliver.
Get in touch to explore clinical and commercial collaboration: partnership@mycardium.com
Thank you for being part of our journey to redefine cardiovascular imaging with AI that clinicians trust.
The MyCardium AI Team
Click here to download this newsletter >