Core Lab Services
MyCardium AI is a medical imaging core lab supporting pharmaceutical sponsors, CROs, and academic institutions conducting international Phase I-IV clinical trials.
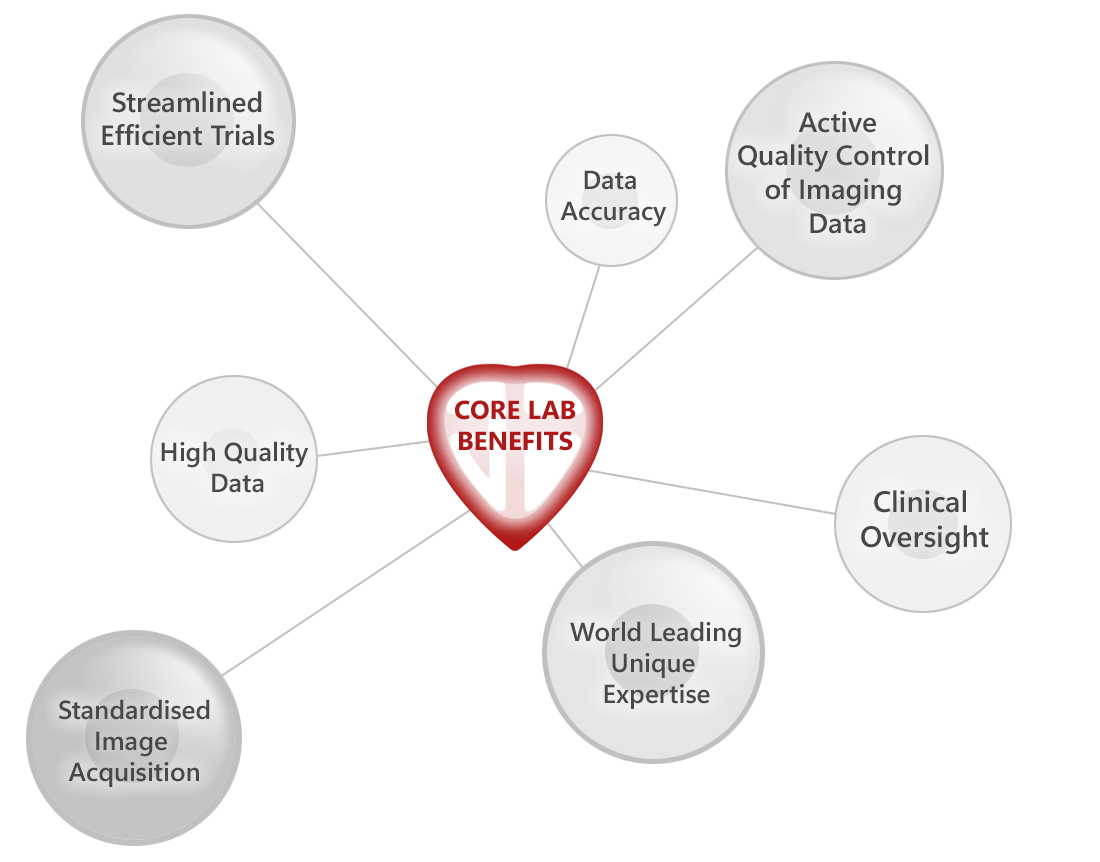
Our Core Lab personnel possess the scientific expertise and project management skills necessary to deliver end-to-end services for cardiac imaging components of clinical trials. Incorporating ISO-27001 and ICH-GCP throughout our workflow ensures regulatory compliance and secure data at all times.
Site Managment
- International Site management
- Global Experience
- On Site training
Study Managment
- Core Lab services supporting Phase I-IV Clinical Trials
- Ensure successful completion of studies
- Highly qualified team
Data Analysis
- Imaging charter
- Delivering accurate, high quality and timely biostatistics
- Planning, Analysis and Reporting
Data Management
- ISO27007: 2013 Certified
- Clean, secure database
- Tailored approach to site logistics
LEARN MORE
We are proud to hold ISO 27001 certification, underscoring our commitment to data security and confidentiality. Additionally, our processes align with ISO 13485 standards, reflecting our dedication to providing safe and effective medical devices. Our adherence to ICH-GCP guidelines ensures that our clinical trial practices meet international ethical and scientific quality standards. These certifications and standards demonstrate our unwavering commitment to regulatory excellence, providing our partners with confidence in the quality and compliance of our services.
Empowering CRO’s with our capabilities
SPECIALISATION IN:
- Amyloidosis (ATTR-CM)
- Fabry’s
- Cardiomyopathies (HCM, DCM, ARVC)
- Heart Failure (HFrEF, HFpEF)
- Myocarditis
- Inflammatory Cardiomyopathies
- Aortic Stenosis
- Cardio-Oncology
- Cell/Gene Therapies
- Cardiac MRI and Echocardiography
Our team offers:
Study Management
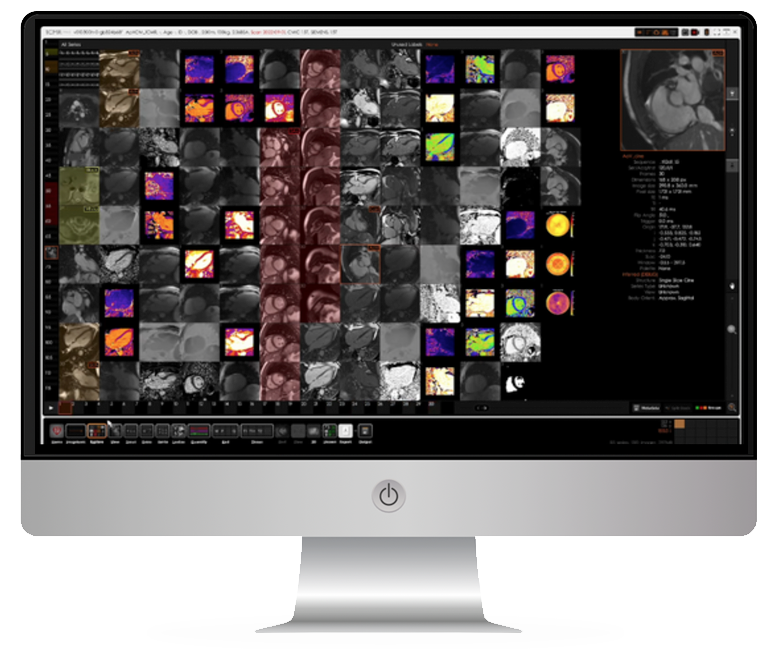
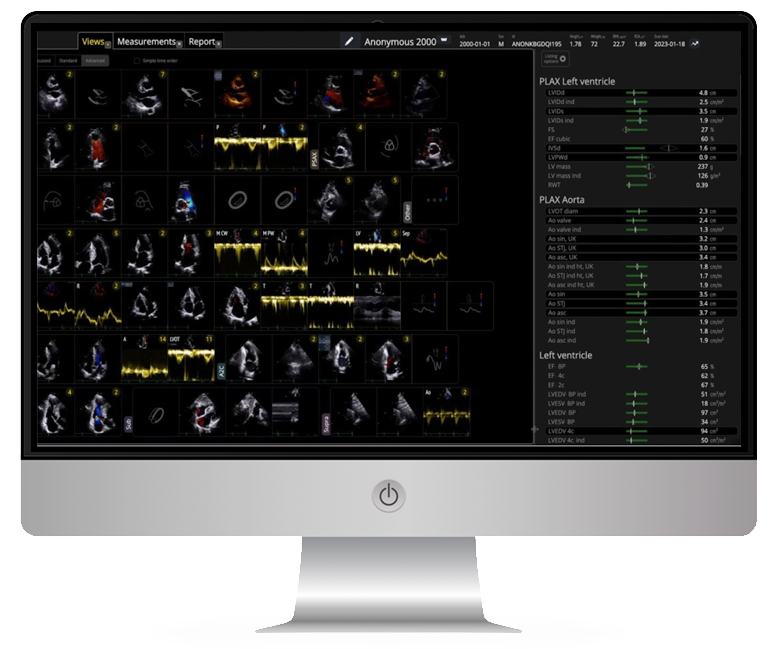
- Cardiac imaging analysis services using our in-house AI software developed for cardiac MRI (CMR) and echocardiography (ECHO).
- Speciality in disease areas such as hypertrophic cardiomyopathy (HCM), amyloid, Fabry’s disease, myocardial infarction, heart failure, and more.
- Expert quality control of CMR and ECHO images by our highly experienced teams of CMR radiographers and ECHO sonographers.
- Medical oversight of CMR and ECHO provided by world-leading expert cardiologists .
- Project management of cardiac imaging aspects of clinical trials, including tracking metrics, site qualification, creation of supporting documentation, and providing a key point of contact for clients.
- High quality data outputs through expert image acquisition guidance, site training, and data management.
Our services include:
Site Management:
- Assistance with site selection due to extensive knowledge and network within cardiac imaging.
- Assessing and qualifying sites for cardiac imaging alongside the sponsor or CRO.
- Able to deep-dive into image acquisition issues and provide tailored advice for optimum image quality.
- Offering on-site or virtual training to participating sites to support image acquisition.
- Quality control of uploaded images and tailored feedback to sites.
- Ongoing support for site technicians for the duration of the study.
Imaging Manual:
- World class experience in CMR and ECHO enables MyCardium to provide the best approach to image acquisition in order to produce optimum quality images.
- Our in-house clinician's create the imaging documentation with site suitability in mind, understanding from their own experience the importance of aligning with the sites’ existing way of working, while incorporating all trial protocol specifications to produce imaging documentation tailored for the highest quality images.
- Our imaging manuals are subject to expert input from world-leading cardiologists.
With 107 active* sites onboarded and over 3820 CMR and 340 Echo images scanned*, MyCardium' s Core Lab is rapidly expanding its global impact, ensuring quality data collection, wherever our partners are.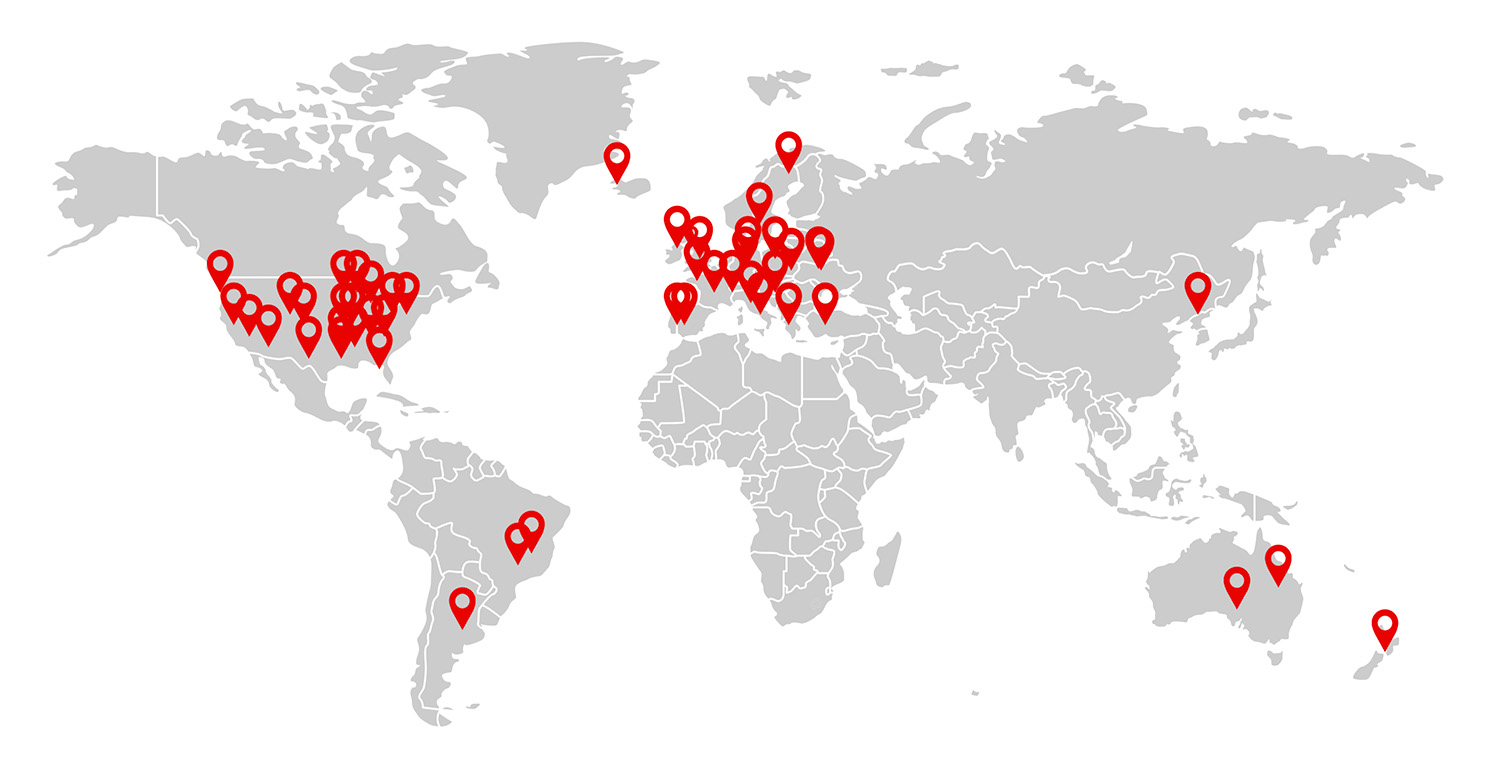
At MyCardium, we’re proud that our Core Lab services consistently earn high satisfaction ratings from our clients. Our Net Promoter Score (NPS) is a key measure of customer loyalty and satisfaction. It reflects the confidence our partners place in us.
An outstanding NPS indicates that clients not only value the quality and reliability of our work but would actively recommend MyCardium AI to others. This is a testament to our commitment to scientific excellence, responsive service, and strong relationships across the industry.
Thank you to all our partners for your trust, we remain dedicated to exceeding expectations every step of the way.
CORE LAB SENIOR TEAM
Click on the team members below for further details.